

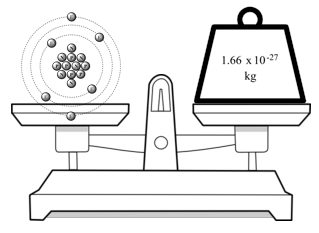
The overall process by which electron(s) are lost or gained is known as ionization. The ‘electron core’ which remains contains two tightly-bound electrons and, because it carries a single net positive charge, is referred to as a monovalent cation. As a result, it is relatively easy to separate this valency electron. The electron in the 2 s-state, usually referred to as the valency electron, is ‘shielded’ by the inner electrons from the attracting nucleus and is therefore less strongly bonded. The next atom, lithium, has a nuclear charge of three ( Z = 3) and, because the first shell is full, an electron must enter the 2 s-state which has a somewhat higher energy. The nucleus of helium contains two neutrons as well as two protons, hence its mass is four times greater than that of hydrogen. These two electrons fill the 1 s-state and will necessarily have opposite spins. In helium, the next element, the nucleus charge is increased by one proton and an additional electron maintains neutrality ( Z = 2). The atom is therefore electrically neutral and for the lowest energy condition, the electron will be in the 1 s-state. The first period commences with the simple hydrogen atom which has a single proton in the nucleus and a single orbiting electron ( Z = 1). The progressive filling of energy states can be followed in Table 1.3. We will now examine the general process by which the Periodic Table is built up, electron by electron, in closer detail. Figure 3.32 shows the optical image of the porous Ta, and Figure 3.33 shows the SEM image of the state of the grain combination in the porous Ta. A macro-reticulated porous Ta foam has been developed by an improved powder-metallurgical process as well, and it has a pore size of 0.5–2.0 mm, interconnected pores, and a porosity of 80%. The disclosed preparation of the porous Ta involves thermal decomposition of polyurethane to a carbon skeleton, and then CVD of Ta on the skeleton to obtain the 3-D, reticulated, porous Ta product. Due to its desirable mechanical strength, elastic modulus, corrosion resistance, and good biocompatibility, porous Ta is applicable to joint implants. For example, a capacitor with porous Ta as the anode has several advantages, including small encapsulation, high capacitance, long lifetime, and high stability. Owing to the properties of Ta, it is widely used in the chemical, metallurgical, electron, electric, and medical industries in applications such as chemical reaction facilities, vacuum furnaces, capacitors, nuclear reactors, aircraft and spacecraft, missiles, and surgery implant materials. Ta is also high corrosion- and wear-resistant, with good biological compatibility. The thermal expansion coefficient for Ta is very small (only 6.6 × 10 − 6 per degree).
Ta is a rare refractory metal with a hardness of HV120 and good ductility. Ta is the sixth period element in the VB family, with an atomic number of 73, relative atomic mass of 180.95, density of 16.6 g/cm 3, and melting point of about 3,000☌ (2,980 ± 20☌), which is a little lower than W and Re.


 0 kommentar(er)
0 kommentar(er)
